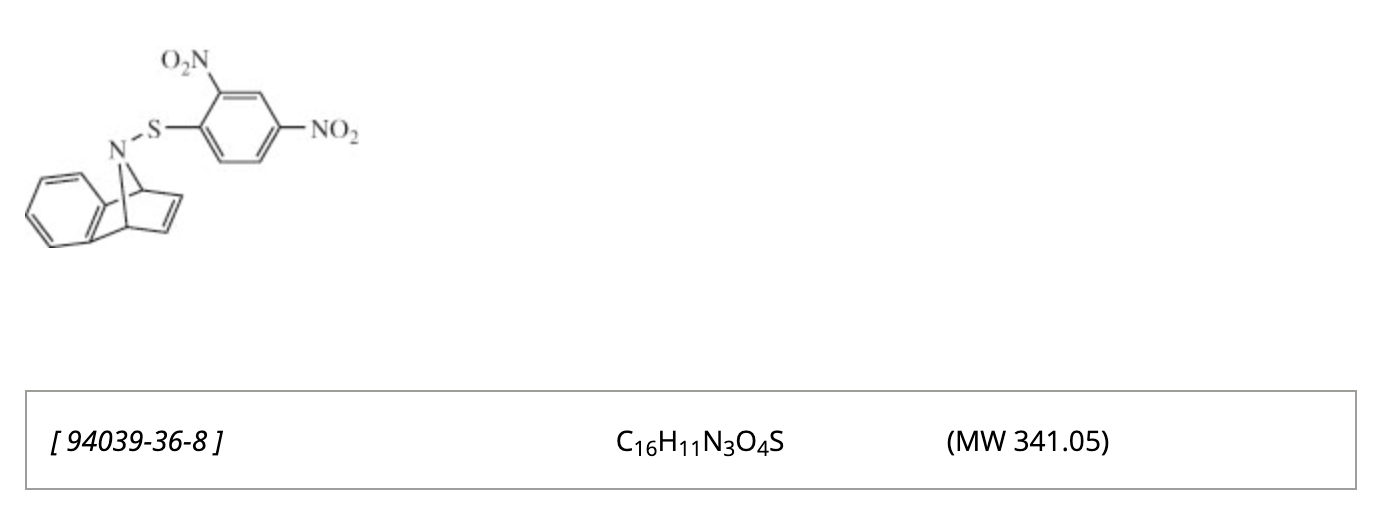
50. Kafle, P; Sharma, I “9-((2,4-Dinitrophenyl)thio)-1,4-Dihydro-1,4-Epiminonaphthalene”, e-EROS, 2026. DOI: 10.1002/047084289X.rn02658
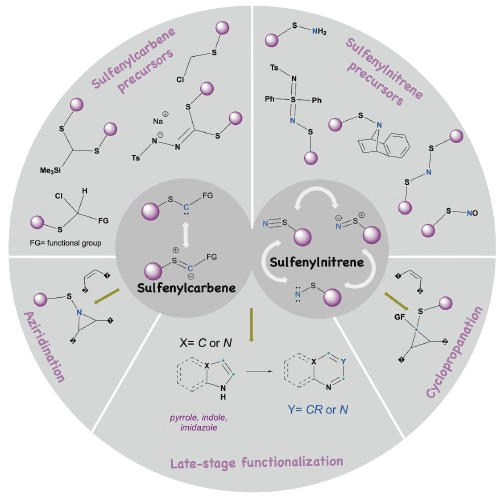
49. Kafle, P; Herndon, D; Sharma, I “Sulfenylcarbenes and Sulfenylnitrenes in Organic Chemistry”, Chem. Soc. Rev., 2025, 54, 10344 - 10362, DOI: 10.1039/d5cs00278h
48. Singh, S.P.; Kharel, P.; Chatterjee, U.; Sharma, I “Harnessing Photochemically Generated Sulfenylnitrene for Stereocontrolled 1,2-cis Glycosylations” ChemRxiv. 2025, DOI: 10.26434/chemrxiv-2025-3429x
47. Kafle, P; Yasuda, S (P.K. and S.Y. contributed equally); Nilson, D; Herndon, D; Fox, R; Sharma, I “Skeletal Editing of Furans into Pyridines”, ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-vplrf
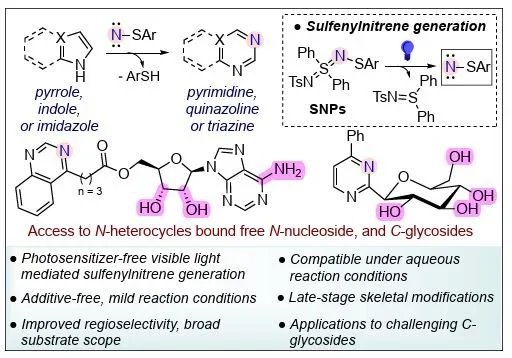
46. Kafle, P; Kharel, P; Nilson, D; Yasuda, Shuhei; Sharma, I “Photolysis-Generated Sulfenylnitrenes Enable Site-Selective Nitrogen Atom Insertion into N-Heterocycles”, Chem, 2025, DOI: 10.1016/j.chempr.2025.102753
45. Singh, S.P.; Chaudhary, U.; Lander, C.; Daróczi, A.; Shao, Y.; Sharma, I “Iron-Carbene-Mediated Catalytic Activation of Conventional Thioglycosides for Stereoselective 1,2- cis-Furanosylations” ACS Catal. 2025, 15, 9886−9896; DOI: 10.1021/acscatal.5c02301
44. Singh S. P.; Chaudhary U.; Daróczi A.; Sharma I.; “Iron-Catalyzed Iodonium Ion Promoted Activation of Conventional Thioglycosides for Stereoselective 1,2-Cis Furanosylations”, Chem. Commun. 2025, DOI:10.1039/D5CC01597A
43. Kafle, P; Kharel, P; Nilson, D; Yasuda, Shuhei; Sharma, I “Blue-Light-Promoted Sulfenylnitrenes for Late-Stage Skeletal Editing of N-Heterocycles: Application to Tethered C-Glycosides and N-Nucleosides”, ChemRxiv, 2025, DOI: 10.26434/chemrxiv-2025-vkfzx
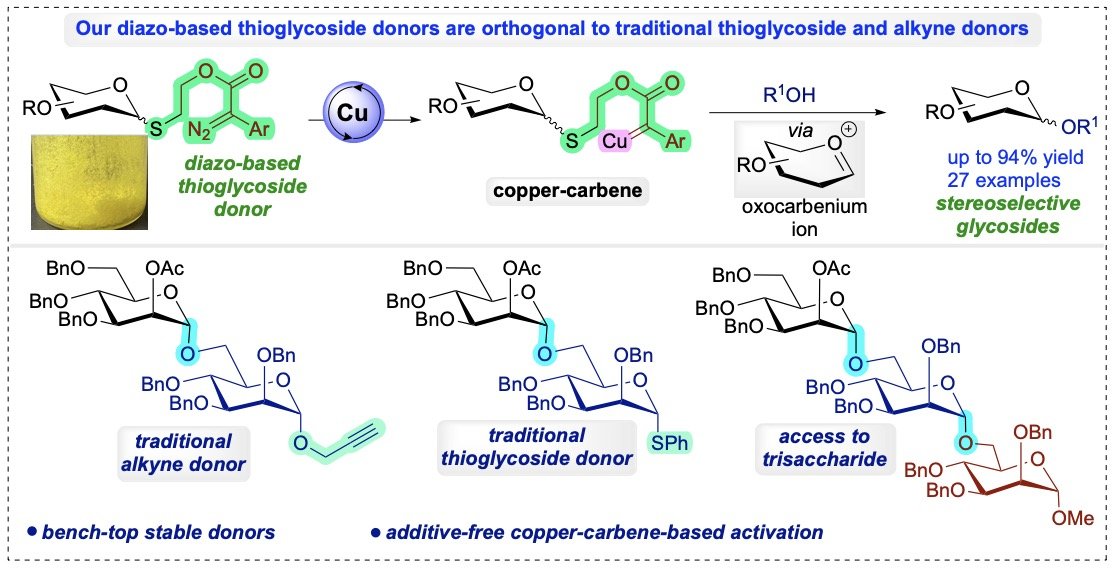
42. Singh, S.P.; Chaudhary, U.; Daróczi, A.; Sharma, I “Fe(OTf)3 or Photosensitizer-free blue light activated diazo-thioglycoside donors for Iterative and stereoselective glycosylations”, Nat. Commun. 2025, 16, 3651, DOI: 10.1038/s41467-025-56445-1
Read the news highlights on our Nature Communication paper in OU News , Phys Org , Science Daily, Bioengineer.org and EurekaAlert!
41. Kafle, P; Herndon, D; Sharma, I “Sulfenylcarbene-Mediated Carbon Atom Insertion for the Late-Stage Functionalization of N-Heterocycles”, JACS, 2025, DOI: 10.1021/jacs.5c02012
40. Ghosh, B; Kafle, P; Mukherjee, R; Welles, R; Herndon, D; Nicholas, KM; Shao, Y; Sharma, I. “Sulfenylnitrene-mediated nitrogen-atom insertion for late-stage skeletal editing of N-heterocycles” Science, 2025, 378(6729), 102-107
DOI: 10.1126/science.adp0974
Read our skeletal editing paper in Science free of charge HERE!
Skeletal editing is making a splash! See the media highlights of our sulfenylnitrene paper in C&EN News, OU News, Phys.org, Norman Transcript, Tech Explorist, News18, EurekAlert!
39. Singh, SP; Herndon, D; Chatterjee, U; Nicholas, KM; Sharma, I. “Investigating the Reactivity of Nitrous Oxide with Hydrazines: Application to the Synthesis of Diarylethanes from Diarylmethylhydrazines” New Journal of Chemistry, 2025, 49, 1182 - 1188 DOI: 10.1039/d4nj04837g
38. Singh, SP; Chuadhary, U; Sharma, I. “Catalytic Thioglycoside Activation with Diazo-Derived Copper Carbenes” Molecules 2024, 29(22), 5367 DOI: 10.3390/molecules29225367
Special Issue in Honor of Prof. David Crich’s 65th Birthday for His Outstanding Contributions to Carbohydrate Chemistry!
37. Ghosh, B; Alber, A; Lander, C.W.; Shao, Y; Nicholas, K.M.; Sharma, I. “Catalytic Activation of Thioglycosides with Copper-Carbenes for Stereoselective 1,2-Cis-Furanosylations” Org. Lett. 2024, 26, 9436-9441 DOI: 10.1021/acs.orglett.4c03281
36. Kafle, P; Herndon, D; Sharma, I. “Inverting Conventional Chemoselectivity of Metal Carbenes with Sulfenylcarbenes for Late-Stage Functionalizations” 2024, ChemRxiv, Preprint Available! DOI: 10.26434/chemrxiv-2024-6dncp
35. Ghosh, B; Kafle, P; Mukherjee, R; (equal contributors BG, PK, RM); Welles, R; Herndon, D; Nicholas, K.M.; Shao, Y; Sharma, I. “Sulfenylnitrene-Mediated Nitrogen-Atom Insertion into Pyrroles, Indoles, and Imidazoles” 2024, ChemRxiv, Preprint Available! DOI: 10.26434/chemrxiv-2024-f80wf-v2
34. Bain, A. I.; Massaro, N. P.; Chinthapally, K.; Sharma, I. “Vinyl Metal Carbene Initiated Cascade Reactions for the Synthesis of Carbo- and Hetero-cycles” 2025 (submitted).
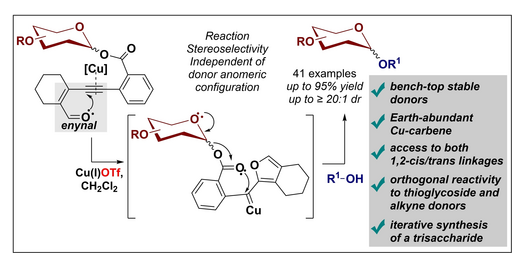
33. Singh, SP; Ghosh, B; (equal contributions SPS, BG) Sharma, I. “Catalytic Orthogonal Glycosylation Enabled by Enynal-Derived Copper Carbenes” Adv. Synth. Catal. 2024 DOI: 10.1002/adsc.202301207
32. Kafle, P.; Ghosh, B.; Hunter, A.; Mukherjee, R.; (equal contributions BG, AH, RM) Nicholas, K. M.; Sharma, I. “Iron-carbene initiated O–H Insertion/Aldol Cascade for the Stereoselective Synthesis of Functionalized Tetrahydrofurans” ACS Catal. 2024, 14, 1292–1299 DOI: 10.1021/acscatal.3c05040
31. Ghosh, B.; Alber, A.; Lander, C.; Shao, Y.; Nicholas, K. M.; Sharma, I. “Catalytic Stereoselective 1,2-cis Furanosylations Enabled by Enynal-Derived Copper Carbenes” ACS Catal. 2024, 14, 1037–1049 DOI: 10.1021/acscatal.3c05237
30. OU, C.; Ghosh, B.; Sharma, I. “A Non-Diazo Approach to Functionalized (2-furyl)-2-pyrrolidines through a Cascade Reaction of Enynal-Derived Zinc Carbenoids with b-Aminoketones” Org. Chem. Front., 2023, 10, 5933 - 5939. DOI: 10.1039/d3qo01354e
29. Bain, A. I.; Chinthapally, K.; Hunter, A. C.; (equal contributions AB, KC, AH) Sharma, I. “Dual Catalysis in Rhodium (II) Carbenoid Chemistry” Eur. J. Org. Chem. 2022 (DOI: 10.1002/ejoc.202101419; Selected for the Front Cover Page).
28. Paymode, D. J.; Sharma, I. Rhodium‐Catalyzed [3+2]‐ANNULATION of Ortho‐ Diazoquinones WITH Enol ETHERS: Diversity‐Oriented Total Synthesis OF AFLATOXIN B2. European Journal of Organic Chemistry 2021, 13, 2034–2040. DOI: 10.1002/ejoc.202100186
27. Schlitzer, S.; Stevens, K.; Sharma, I. “A Metal Free Aromative Cascade for the Synthesis of Diverse Heterocycles” Org. Chem. Front. 2020 (DOI: 10.1039/c9qo01336a; Selected for the Front Cover Page).
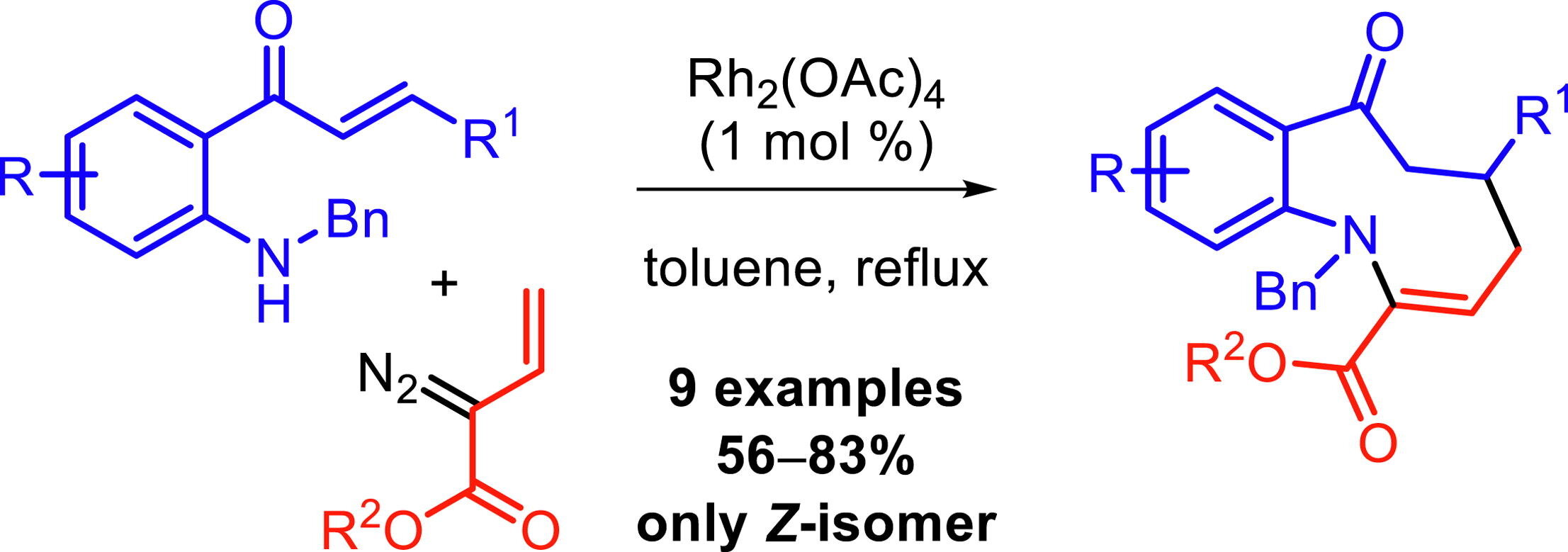
26. Chinthapally, K.; Massaro, N.; Sabrina, T.; Gardner, E.; Sharma, I. “Trapping Rhodium Vinylcarbenoids with Aminochalcones for the Synthesis of Medium-Sized Azacycles” Tetrahedron Letters 2019, 60, 151253. DOI: 10.1016/j.tetlet.2019.151253
25. Massaro, N.; Chatterji, A.; Sharma, I. “A three Component Approach to Pyridine Stabilized Keteneimines for the Synthesis of Diverse Heterocycles” J. Org. Chem. 2019, 84, 13676–13685. DOI: 10.1021/acs.joc.9b01906
24. Hunter, A. C.; Chinthapally, K.; Bain, A.; Steven, J. C.; Sharma, I. "Rh/Au Dual Catalysis in Carbene sp2-CH Functionalization/Conia-ene Cascade to the Stereoselective Synthesis of Diverse Spirocarbocycles” Adv. Synth. Catal. 2019, 361, 2951–2958. DOI: 10.1002/adsc.201900079
23. In collaboration with NIH-NCATS Library Screening Program: "Canvall: a Crowd-Sourced Natural Product Screening Library for Exploring Biological Space” ACS Cent. Sci. 2018, 4, 1727–1741. DOI: 10.1021/acscentsci.8b00747
22. Massaro, N. P.; Stevens, J. C.; Chatterji, A.; “Stereoselective Synthesis of Diverse Lactones through Diverted Carbene Ketoacid Insertion Reactions” Org. Lett. 2018, 20, 7585–7589. DOI: 10.1021/acs.orglett.8b03327
21. Hunter, A. C.; Almutwalli, B.; Bain, A.; Sharma, I. "Trapping Rhodium Carbenoids with Aminoalkynes for the Synthesis of Diverse N-Heterocycles” Tetrahedron 2018, 74, 5451–5457. DOI: 10.1016/j.tet.2018.06.042 (Invited article in honor of Derek Barton on his centennial birthday).
20. Hunter, A.C.; Schlitzer, S.C.; Stevens, J.C.; Almutwalli, B.; Sharma, I. “A Convergent Approach to Diverse Spiroethers through Stereoselective Trapping of Rhodium Carbenoids with Gold Activated Alkynols” J. Org. Chem. 2018, 83, 2744–2752. DOI: 10.1021/acs.joc.7b03196
19. Chinthapally, K.; Massaro, N.; Padgett, H.L.; Sharma, I. “A Serendipitous Cascade of Rhodium Vinylcarbenoids with Aminochalcones for the Synthesis of Functionalized Quinolines” Chem. Comm. 2017, 53, 12205–12208. DOI: 10.1039/C7CC07181G
18. Chinthapally, K; Massaro, N. P.; Sharma, I. “Rhodium Carbenoid Initiated O–H Insertion/Aldol/Oxy-Cope Cascade for the Stereoselective Synthesis of Functionalized Oxacycles” Org. Lett. 2016, 18, 6340–6343. DOI: 10.1021/acs.orglett.6b03229
17. Hunter, A. C.; Schlitzer, S. C.; Sharma, I. “Synergistic Diazo–OH Insertion/Conia-Ene Cascade Catalysis for the Stereoselective Synthesis of γ-Butyrolactones and tetrahydrofurans” Chem. Eur. J. 2016, 22, 16062–16065 DOI: 10.1002/chem.201603934.
16. In Collaboration with Professor Lakshmi Devi (Mount Sinai, New York) and Joseph Parello (Vanderbilt University); Gupta, A.; Gomes, I.; Bobeck, E. N.; Fakira, A. K.; Massaro, N. P.; Sharma, I.; Cave, A.; Hamm, H. E.; Parello, J.; Devi, L. A. “Collybolide is a Novel Biased Agonist of κ-Opioid Receptors with Potent Antipruritic Activity” Proc. Natl. Acad. Sci. 2016, 113, 6041–6 DOI: 10.1073/pnas.1521825113
15. Hunter, A. C.; Chinthapally, K.; Sharma, I. “Rh2(esp)2: An Efficient Catalyst for O–H Insertion Reactions of Carboxylic Acids into Acceptor/Acceptor Diazo Compounds” Eur. J. Org. Chem. 2016, 2260–2263, selected for the front cover page; DOI: 10.1002/ejoc.201600064
Publications (Prior to Independent Career)
14. Ji, C.; Sharma, I.; Pratihar, D.; Hudson, L. L.; Maura, D.; Guney, T.; Rahme, L. G.; Pesci, E. C.; Coleman, J. P.; Tan, D. S. “Designed small-molecule inhibitors of the anthranilyl-CoA synthetase PqsA block quinolone biosynthesis in Pseudomonas aeruginosa” ACS Chem. Biol. 2016, 11, 3061–3067. DOI: 10.1021/acschembio.6b00575
13. Matarlo, J. S.; Evans, E. C.; Sharma, I.; Lavaud, L. J.; Ngo, S. C.; Shek, R.; Rajashankar, K. R.; French, J. B.; Tan, D. S.; Tonge, P. J. “Mechanism of MenE Inhibition by Acyl-Adenylate Analogues and Discovery of Novel Antibacterial Agents” Biochemistry 2015, 54, 6514–6524. DOI: 10.1021/acs.biochem.5b00966
12. Sharma, I.; Wurst, J.; Tan, D. S. “ Solvent-Dependent Divergent Functions of Sc(OTf)3 in Stereoselective Epoxide-Opening Spiroketalizations” Org. Lett. 2014, 16, 2474–2477. DOI: 10.1021/ol500853q
11. In collaboration with Dr. Susruta Majumdar (Pasternak Lab, MSKCC), Váradi, A.; Palmer, T. C.; Notis, P. R.; Redel-Traub, G. N.; Afonin, D.; Subrath, J. J.; Pasternak, G. W.; Hu, C.; Sharma, I.; Majumdar, S.; “Three-Component Coupling Approach for the Synthesis of Diverse Heterocycles Utilizing Reactive Nitrilium Trapping” Org. Lett. 2014, 16, 1668–1671. DOI: 10.1021/ol500328t
10. Sharma, I.; Tan, D. S. News and Views “Drug Discovery Diversifying Complexity” Nature Chemistry 2013, 5, 157–158. DOI: 10.1038/nchem.1581
9. Lu, X.; Zhou, R.; Sharma, I.; Li, X.; Kumar, G.; Swaminathan, S.; Tonge, P. J.; Tan, D. S. “Stable Analogues of OSB-AMP: Potent Inhibitors of MenE, the o-Succinylbenzoate-CoA Synthetase from Bacterial Menaquinone Biosynthesis” ChemBioChem. 2012, 13, 129–136. DOI: 10.1002/cbic.201100585
8. Sharma, I.; Bohe, L.; Crich D. “Influence of Protecting Groups on the Anomeric Equilibrium; Case of the 4,6-O-Benzylidene Acetal in the Mannopyranose Series” Carbohydr. Res. 2012, 357, 126–131. DOI: 10.1016/j.carres.2012.05.025
7. Sharma, I.; Crich D. “Direct Fmoc-Chemistry-Based Solid Phase Synthesis of Peptidyl Thioesters” J. Org. Chem. 2011, 76, 6518–6524. DOI: 10.1021/jo200497j
6. Aubry, S.; Sasaki, K.; Sharma, I.; Crich, D. "Influence of protecting groups on the reactivity and selectivity of glycosylation: Chemistry of the 4,6-O-benzylidene protected mannopyranosyl donors and related species" Topics Curr. Chem. 2011, 301, 141–188. DOI: 10.1007/128_2010_102
5. Crich, D.; Sharma, I. “Influence of the O3 Protecting Group on Stereoselectivity in the Preparation of C-Mannopyranosides with 4,6-O-Benzylidene Protected Donors” J. Org. Chem. 2010, 75, 8383–8391. DOI: 10.1021/jo101453y
4. Crich, D.; Sharma, I. “Triblock Peptide and Peptide Thioester Synthesis with Reactivity-Differentiated Sulfonamides and Peptidyl Thioacids” Angew. Chem. Int. Ed. 2009, 48, 7591–7594. DOI: 10.1002/anie.200903050
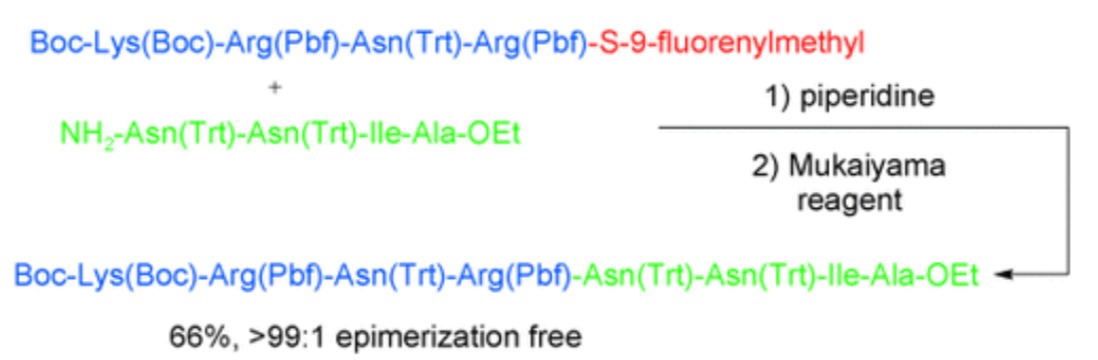
3. Crich, D.; Sharma, I. "Epimerization-Free Block Synthesis of Peptides from Thioacids and Amines with Sanger’s and Mukaiyama’s Reagents” Angew. Chem. Int. Ed. 2009, 48, 2355–2358. DOI: 10.1002/anie.200805782
2. Crich, D.; Sharma, I. “Is Donor-Acceptor Hydrogen Bonding Necessary for 4,6-O-Benzylidene Directed β-Mannopyranosylation. Stereoselective Synthesis of β-C-Mannopyranosides and α-C-Glucopyaronosides” Org. Lett. 2008, 10, 4731–4734. DOI: 10.1021/ol8017038
1. Mal, D.; Ray, S.; Sharma, I. "Direct Access to 1,4-Dihydroxyanthraquinones: The Hauser Annulation Reexamined with p-Quinones” J. Org. Chem. 2007, 72, 4981–4984. DOI: 10.1021/jo062271j