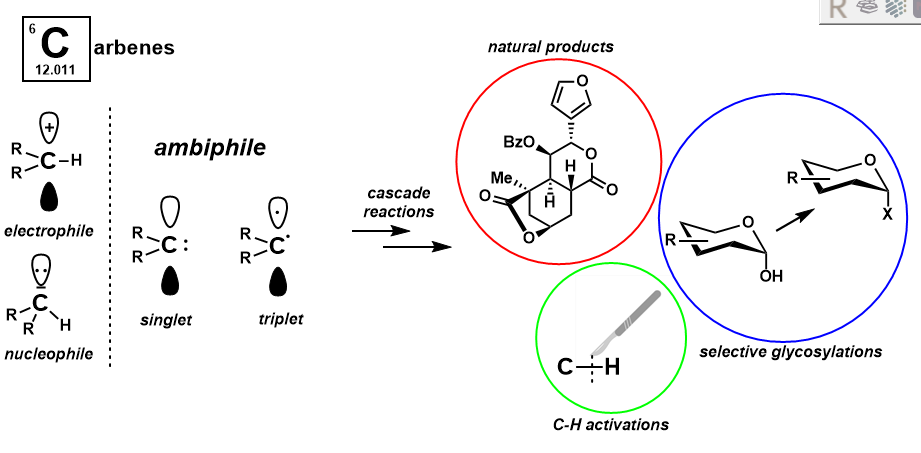
Carbenes are six-electron carbon complexes possessing both a lone pair of electrons and an empty p-orbital. This implies that carbenes are able to function as both a nucleophile and an electrophile, making them ambiphilic reactive intermediates. As such, they are well primed for multistep cascade reactions in which multiple bonds are sequentially formed. Our group applies carbene chemistry to challenging problems in organic synthesis, such as stereoselective glycosylation, C-H activation, and the synthesis of complicated molecules. Further, our group is interested in benchtop stable carbene precursors that do not rely on traditional carbene sources such as diazo compounds.
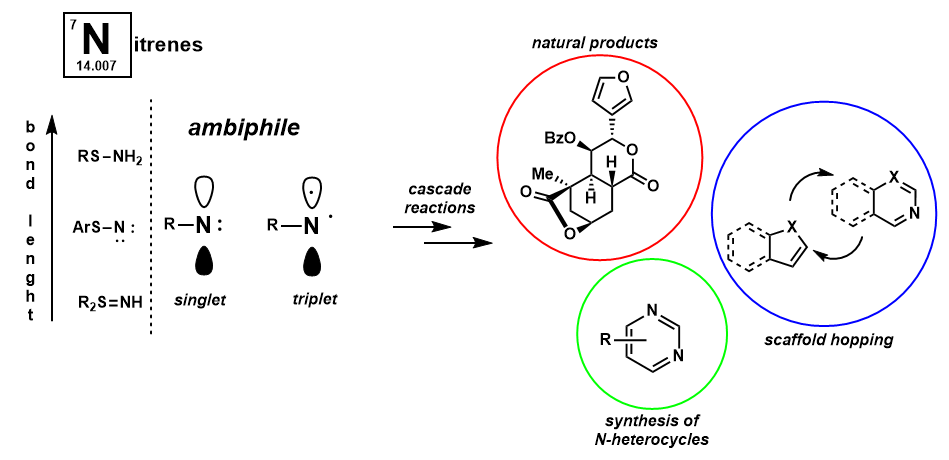
Nitrenes are the isoelectronic, nitrogen-based cousins of carbenes. Again, these are six-electron ambiphilic reactive intermediates which are primed for cascade reactions. Specifically, our lab is interested in sulfur-based nitrene precursors which have been shown to generate nitrenes under thermal, oxidant-free, additive free conditions. Nitrenes may be used in the synthesis of a myriad of important nitrogen-containing heterocycles, including the construction and late-stage functionalization of biologically relevant molecules.
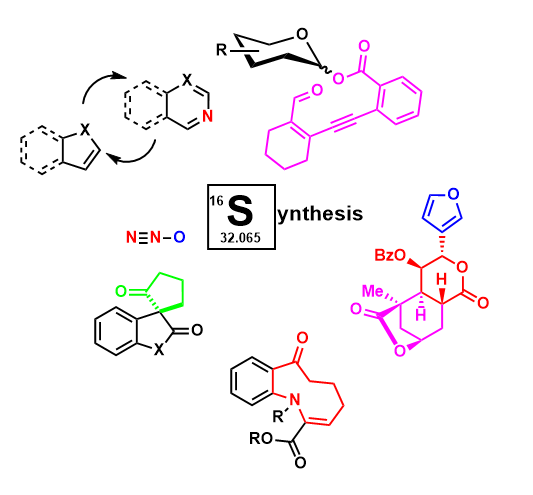
Sharma Laboratories is a synthetic organic laboratory focusing on method development, especially the discovery of novel chemical reactions involving reactive intermediates. Beyond our work with carbene and nitrenes, we are interested in the synthetic organic utility of nitrous oxide, the synthesis and activity-based study of biologically active compounds, and the design of Earth-abundant metal catalysts.
“A method is more important than a discovery, since the right method will lead to new and even more important discoveries” -Lev Landau
“Chemical synthesis is uniquely positioned at the heart of chemistry, the central science, and its impact on our lives and society is all pervasive” - E.J. Corey